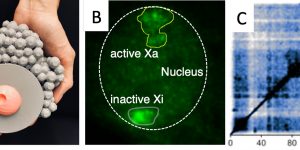
Epigenetic mechanisms control interpretation of the genome, which is identical between cell types. The inactivation of one of the two X-chromosomes in female mammals is a prominent example of such epigenetic regulation, in which gene expression from an entire X-chromosome is suppressed to avoid gene dosage imbalance with males, which only have a single X.
In our group, we study the mechanisms of X-chromosome reactivation, which reverses X-inactivation. We make use of in vivo and in vitro approaches to identify new regulators of this epigenetic reprogramming process. Our goal is to establish how the different steps (e.g. changes in 3D-chromatin structure, epigenetic marks, X-linked gene reactivation) are achieved and how they are linked to their biological context within pluripotent cells and in the germ line.
Furthermore, we are studying in collaboration with the fertility Clinic Eugin, what effects age has on epigenetic mechanisms affecting the quality of human oocytes. With this research, we want to apply our expertise in germ cell development and epigenetics to address clinical outcomes of reproductive aging.